This page present information about the research project FRVS 1749/2002
Study of physico-mineralogical processes in slicate binder structure during hydration
Ing. Jiri Zach
BRNO University of Technology
Faculty of civil engineering
The Influence of the Chemical Admixtures on Hydration Process of Portland Cements
All chemical admixtures added to concrete in order to improve the workability in fresh state and to improve its final properties, have a significant influence on the reaction kinetics of hydration. The knowledge of this effect is very important not only for the optimization of a cement mixture but even for the optimal placing of concrete process. Considering the fact the clinker minerals hydration is an intensive exothermic reaction, it is possible to control this process by means of intensity measurement of hydration heat development.
1. Introduction
The cement is a hydraulic binder mostly produced from finely ground mixture of Portland clinker and a certain quantity of hydraulic admixtures and additives controlling the hydration velocity (gypsum).
The hydraulic ability is the property of some binders to react with water under formation of insoluble compounds, which don’t decompose but which in course of time increase in strength.
In hydraulic binders the particles of hydraulic products are bond by primary valence forces. These forces are much stronger than absorptive forces binding the water molecules to the surface of particles. The particles of hydration products have to form a continuous skeleton - a continuous irregular lattice bound by primary valences.
The carriers of hydraulic properties are the clinker minerals. The main clinker minerals are presented in the next table.
Table 1: Survey of main clinker minerals and their presence in Portland clinker:
|
Mineral type |
Chem. composition |
% in p-clinker |
|
silicates |
C3S |
40 – 60 % |
|
C2S |
10 – 40 % |
|
|
aluminates |
C3A |
7 – 18 % |
|
ferrites |
C4AF |
8 – 18 % |
Tricalciumsilicate (C3S) - is the main carrier of strength and it is present in the highest extent. It is mostly the high temperature a-C3S stabilized by solid solutions of C3A. In contains even small amounts of Al2O3, MgO, FeO, Fe2O3 and it is termed as Alit. It is formed at temperatures higher than 1350°C by reaction of C2S with free lime, and it is characterized by high reactivity with water demonstrated by a fast rate of strength increase especially concerning initial strength and by a big hydration heat development (500 kJ.kg-1in 28 days).
Dicalciumsilicate (C2S) – is the carrier of long-term strength values. It is formed by direct reaction of CaO and SiO2 at the temperature of 1000°C till 1250°C. It is after C3S the second most important mineralogical component of P-cement. It exists in four modifications a, a’, b, g. In Portland cement mostly the b-form is present (termed as Belit) and sometimes even the g-form. The a-form is stable at temperatures higher than 1420 till 1450°C. In the case of slow cooling it is transformed in modifications a’ and b. In the case of very slow cooling at the temperature of 675 till 525°C the modification g is formed which has a very small hydraulic power. Between mentioned modifications the highest hydraulic power has the b-C2S a mineral characterized mostly by regular rounded grains. The hydration heat of C2S is the lowest from all clinker minerals (250 kJ.kg-1 in 28 days). The development of this heat is slow.
Tricalciumaluminate (C3A) – is formed by reaction of CaO and Al2O3 at the temperature 1250 till 1300°C. It nearly immediately sets with water. In practice the rate of hydration is controlled by addition of gypsum in the quantity of about 5 % in relation to clinker mass. During the hydration of C3A a strong heat development takes place (860 – 1090 kJ.kg-1 in 28 days). C3A is first of all the carrier of initial strength but with respect to modification changes of C3A hydration products and to their volume changes the C3A content in cement has to be reduced. The hydration heat is up to 1350 kJ.kg-1 in 28 days.
Aluminoferrites (C6AxFy) - are formed at the temperature of 1200 – 1300°C. The ferric component is present in clinker in form of mixed crystals with the composition C6AxFy.
They take part on strength increase of P-cement especially in the initial phase. The typical representative of aluminoferrites is Brownmillerit C4AF. The heat development during the hydration is about 420 kJ.kg-1 in 28 days. During reaction with water they set quickly but the strength increases more slowly and its value is lower in comparison with the former mentioned clinker minerals. The content of aluminoferrites fluctuates mostly between 8 % and 18 %
2. Cement hydration
As already described practically every cement contains besides P-clinker a certain quantity of admixtures the quantity of which is mostly > 8%. Therefore already the cement reaction with water alone (following the cement composition) significantly differs from the reaction of pure clinker. During the manufacture of concrete mixtures chemical agents and admixtures are added to cement which influence the rheological and final properties of concrete.
Each additive or admixture addition has a certain influence on hydration kinetics of the concrete mixture. Generally the substances added to concrete can be divided as follows:
Physico-chemically active substances – admixtures:
- chemical admixtures reacting with binder particles
- chemical admixtures influencing the binder solubility in water
- chemical admixtures changing the electrostatic potential of the mixture
Physically active components – admixtures:
- change of heat conductivity of the mixture
- change of heat capacity of the mixture
- solid particles surface change of the mixture components
Considering the fact that the hydration kinetics itself depends on many external and internal factors the empirical description of individual processes is very complicated. An effective possibility to influence the hydration process of the concrete mixture seems to be the determination of hydration heat development in izoperibolic calorimeters.
3. The influence of the chemical admixtures on hydration process of PC
The objective of the research, realized at Brno University of Technology, Faculty of Civil Engineering is to formulate a general description of the effect of chemical additives on the hydration process of silicate binder mixtures. During the research calorimetric measurements were performed in particular of cement pastes modified by the addition of different chemical additives from different building chemistry manufacturers. In order to enable the easier comparison of measured values a uniform cement of class CEM I 42,5 R was used (one manufacturer). The measurements were performed in a set of isoperibolic calorimeters, which were developed for this purpose within the research project. The resulting courses of hydration heat development (temperatures) were continuously registered by a PC-unit and they were further evaluated (hydration heat). In the framework of the research the addition effect of more types of accelerating, retarding and plasticizing additives was checked.
In these groups always more types of additives were tested, which differed from each other by its chemical base and by the way of physico-chemical effects.
4. Accelerating and retarding admixtures
Additives controlling the hydration kinetics have been known already in the past century. From the point of view of chemical regulating admixtures it is necessary do respect the fact that this additives react with different components of Portland cement clinker in different way. For this reason it is necessary during laboratory test strictly separate the problem of setting from the problem of hardening. This means that from the view of reaction kinetics the additive can react only with a definite component of Portland cement and this means the setting of the mixture but the hydration of the greatest portion of clinker minerals can take place later. In a series of laboratory tests it was proved that by using some chemical admixtures begin of the hydration process can be postponed by dozens of hours.
The main types of accelerating additives used in the Czech Republic in the year 2002 are:
- calcium formate
- nitrates and nitrites of alkaline metals and alkaline earth metals (calcium- Ca(NO3)2, sodium - NaNO3, NaNO2, potassium – KNO3, KNO2)
-thiocyanates (sodium – NaSCN and calcium Ca(SCN)2 )– this group is according to the previous term often called rhodanides.
- chlorides CaCl2
Accelerating admixtures are mostly used with concreting in winter and in the production of prefabricated elements in order to accelerate the cycles of production. This additives act in the binder mixture as electrolytes, and they regulate the hydration process of individual Portland clinker components. The oldest used additive is the solution of calcium chloride, which has a positive influence especially on the hydration of aluminates components of clinker. The use of calcium chloride was step by step reduced because the chloride ions have a negative influence on reinforcement. In the next phase additives based on nitrates and nitrites were applied. Especially the nitrites had a positive effect concerning the inhibition of reinforcement corrosion. Further additives are based on thiocyanites and alkaline salts of organic acids.
|
|
|
|
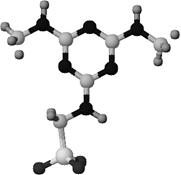
Fig. 1: Structural picture of calcium salt of formic acid |
Fig. 2: Structural picture of calcium and sodium thiocyanate |
Basic types of retardation admixtures used in the Czech Republic in the year 2002:
- phosphates (sodium, potassium) K3PO4, Na3PO4
- lignosulphonates
- saccharides and polysaccharides
- mixtures of above mentioned additives and plasticizing additives with retarding effect(see below)
|
|
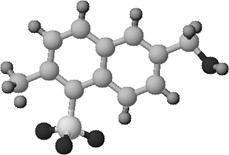
Fig.3: Structural picture of a saccharose unit |
From the point of view of chemical additives effect on the reaction kinetics of Portland clinker all additives can be designated as retardation additives because in thumping majority they cause certain retardation (delay) of hydration reaction. The retarding agents mostly affect the crystalline-chemical and physical processes during the course of hydration and they form on the surface of cement particles badly soluble calcium salts. The retarding agents are mostly organic and inorganic groups containing hydroxyl and carboxyl groups.
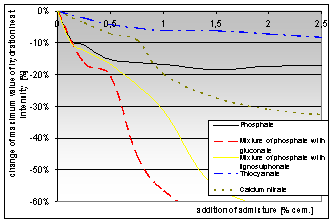
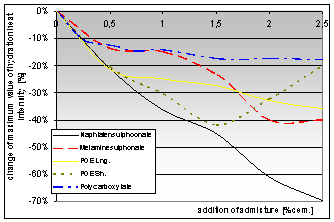
According the test results of cement pastes (CEM I 42,5 R) modified by the addition of above mentioned additives hydration, heat observation graphs were constructed. These graphs show the change of maximum intensity of hydration heat generation with samples modified by different kinds of chemical additives in dependence on the dose of the additive.
|
|
Graph 1: Dependence of the maximum value of hydration heat intensity change on the addition of chemical admixtures |
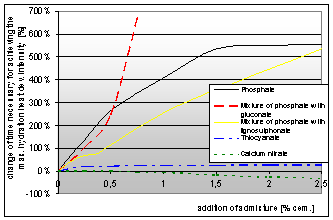
The following graph represents the change of time necessary to achieve the maximum value of hydration heat development. This time corresponds with the hydration begin displacement in the case of samples modified by admixtures in dependence on the dose of the admixture.
|
|
Graph 2: Dependence of the time necessary for achieving the maximum hydration heat development intensity on the addition of chemical admixtures |
5. Plasticizers
The plasticizing additives are added to concrete in order to improve the rheological properties in fresh state and to decrease the necessary volume of batch water. This leads to improvement of physico- mechanical properties of resulting concrete. According to the mode of action and its effectiveness the plasticizing and the superplasticizing admixtures can be divided into three groups:
- surface-active admixtures,
- admixtures covering the cement grains, causing the repulsion of grains based on electrostatic potential,
- admixtures covering the cement grains, causing the repulsion of grains based on steric hindrance.
The basic types of plasticizing agents used in the Czech Republic in the year 2002 are:
- lignosulphonates
- melaminesulphonates
- naphtalensulphonates
- (Copolymers of acrylic esters)-polyacrylates
- polyoxyethethylens
- polycarboxylates
|
|
|
|
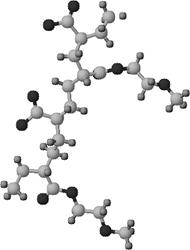
Fig. 4: Structural picture of simplified lignosulphonate polymer unit |
Fig.5: Structural picture of simplified melaminesulphonate polymer unit |
|
|
|
|
Fig. 6: Structural picture of simplified naphthalensulphonate polymer unit |
Fig.7: Structural picture of simplified polycarboxylate polymer unit |
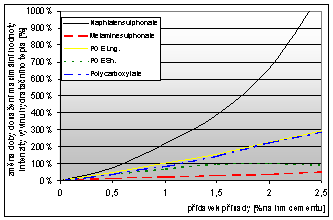
The following graphs show the results of hydration heat development intensity of samples modified by different types of plasticizing additives in dependence on the dose of the additive.
|
|
Graph 3: Dependence of the maximum value of hydration heat intensity change on the addition of chemical admixtures (POE - polyoxyethethylens, Lng. – long polymer, Sh. – short polymet) |
|
|
Graph 4: Dependence of the time necessary for achieving the maximum hydration heat development intensity on the addition of chemical admixtures (POE - polyoxyethethylens, Lng. – long polymer, Sh. – short polymet) |
6. Conclusion
From graphs 1 – 4 it is evident that the reactive kinetics of binder in the concrete mixture is influenced by practically arbitrary type of chemical admixture. Further following the laboratory measurements it was determined, that some types of plasticizing agents have a retarding effect, on the one hand decreasing the hydration heat development intensity, on the other hand delaying the begin of hydration. In the case of retarding agents we determined that some types of agents only delay the begin of hydration and other have an influence even on the hydration intensity. A very interesting fact was observed in the case of accelerating agent based on calcium nitrate because this additive accelerates the begin of hydration but in a significant way decreases its intensity.
The knowledge of the extent to effect the reaction kinetics by chemical agents is of importance particularly for placing of concrete in massive structures. It is also of importance in types of structures where an effort is made to eliminate tensile stresses, which can be formed possibly by inadequate temperature increase in the massive material during the initial period after placing of concrete. At the same time it was demonstrated that by the selection of an adequate chemical agent it is possible to influence the hydration very effectively in order to eliminate the greater part of these negative effects.
7. Other references
1. Stastnik, S., Zach, J. Influence of plasticizing admixtures in concrete on hydratation heat liberation proces of cement, World Conference On Concrete Materials and Structures, Malaysia 2002, ISBN 983-9414-33-X
2. Stastnik, S., Zach, J., Heat technical properties of concrete, 3rd International conference of concrete and concrete structure, Zilina 2002, Slovakia, ISBN 80-7100-954-7
3. Stastnik, S., Zach, J. Influence of plasticizing admixtures in concrete on hydratation heat liberation process of cement, I. International conference Non-traditional Cement & Concrete University of Technology Brno, Czech Republic, ISBN 80-214-2130-4